Nutrition Powder Plant
INTEGRATING MULTIPLE CONTROL SYSTEMS
The Challenge
The project entailed archiving and synchronizing the complete process data with a global remote server. A crucial requirement was the implementation of batch control and recipe management, while also incorporating powder blending and packaging systems. Furthermore, compliance with the US FDA-approved 21CFRpart 11 system was an essential aspect of the project.
The Solution
The pharmaceutical industry presents captivating automation challenges with demanding and intricate requirements.
In the pharmaceutical industry, exploring the Operator system presents opportunities to engage with recipe management, historical trends, batch management, user login, audit trail, and more. The Siemens DCS system holds a prestigious reputation and is widely adopted by the pharmaceutical industry, presenting a significant advantage for both the customer and Velox.
Velox Automation, actively involved in the pharmaceutical industry, collaborates with both OEM projects and end-users to deliver innovative automation solutions. Leveraging our experience, we offer comprehensive solutions that encompass process automation, FDA compliance, and seamless integration of process data with global setups.
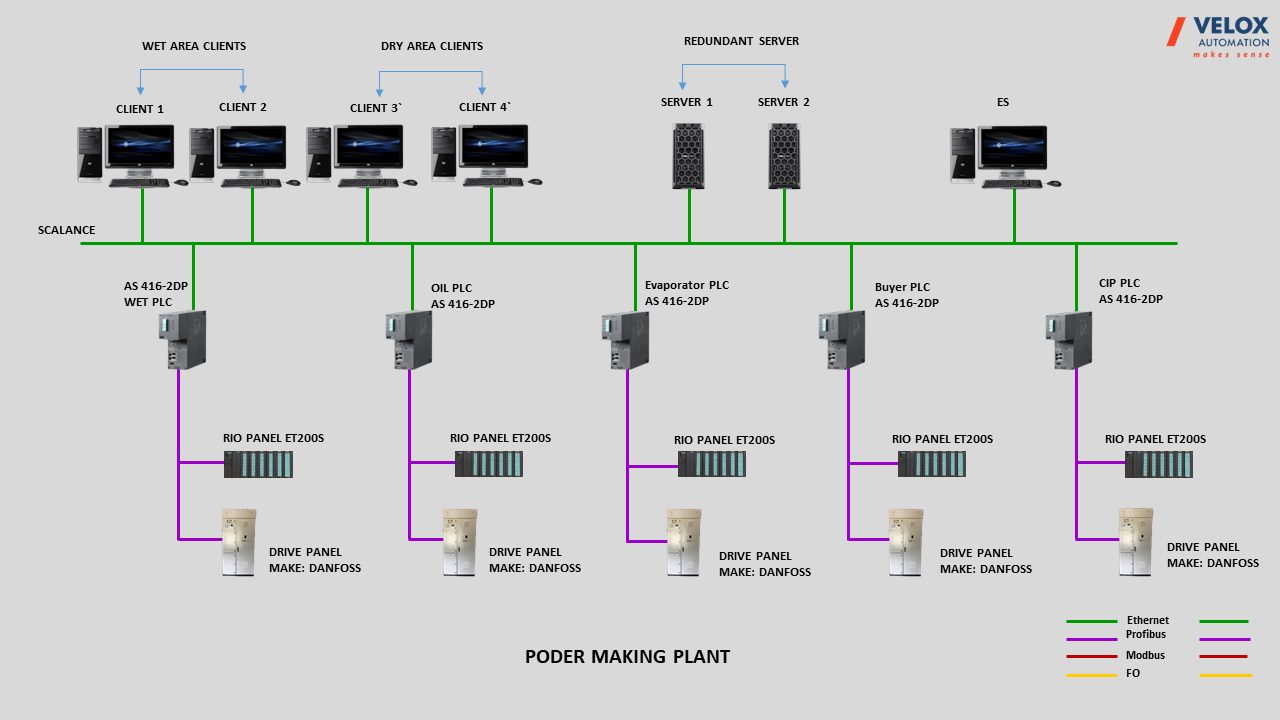
- Six PCS7 controllers were deployed, each dedicated to specific systems like WET, OIL, DRY, EVAP, and CIP, ensuring precise and efficient control.
- I/Os were distributed across the plant, connected to area controllers via Profibus, enabling effective communication and control in respective areas.
- A pair of Redundant servers was formed based on PCS7 OS server with 04 operator clients for WET process operation.
- A separate PC was dedicated to recipe management for the entire plant, streamlining the process and enhancing operational efficiency.
- The project involves a DCS system for a complete powder manufacturing plant, encompassing both wet and dry systems for streamlined control and operation.
- Powder blending and packaging systems were also part of the project.
- The project's foremost requirement was implemented, of batch control and recipe management, along with the adoption of the US FDA-approved 21CFRpart 11 system for regulatory compliance.
- Process data was archived and synchronized with a global remote server for seamless accessibility and data integrity.
Why Velox Solutions Makes Sense
We are excited to receive inquiries related to motion control applications, as our specialized expertise enables us to deliver comprehensive solutions in this field.
- The entire system was locally developed and commissioned
- Our programming standards aligned seamlessly with the customer's global standards, guaranteeing compatibility and consistency.
- The system achieved complete automation and US FDA compliance.
- Data management with global servers was established.
- The Indian plant was seamlessly linked to the customer's global setup, providing access to best batch data for improved operations.
- Utilizing energy-efficient machinery and production methods helps conserve resources and decrease carbon emissions
- Good environmental impact can be achieved by implementing sustainable practices throughout the supply chain, responsibly sourcing ingredients, reducing transportation emissions and partnering with responsible suppliers.
- Minimizing food waste will contribute positively to the environment.